Yeasts appear in our life in many forms: the yogurt we drink, the bread we eat, the beer we love. We cannot live without these magnificent creatures, even though we cannot directly see them with our naked eyes.
But yeast matters even more than we think: currently, scientists are using special strains of yeast to develop drug targets that might be the key to discovering new treatments to fight human cancers. Why yeast?
The simplicity of yeast cells makes them a suitable tool for modern scientists to genetically engineer, study, and gain insights about human cells. More importantly, we bear disturbing similarities with yeasts. For example, the cell structure of yeast is almost identical to that of human’s cell but simpler.
Besides, yeasts also have two different genders: MATa and MATα (like male and female), just like us. But, because they do not have eyes, they have to find their “soul mates” with pheromones—a or α factor (chemicals released into the environment to send information).
During sexual reproduction, both MATa and MATα need to be physically in touch with each other for the exchange of genetic information. To locate the other type, yeasts can sense the pheromone produced by the opposite sex.
After knowing the existence of their partner, yeasts will extend toward the other type through a series of reactions including sensing, passing, and responding to the outside signals. Scientists can use those characteristics to artificially activate or deactivate the signaling pathways.
For example, in the experimental setting, we trick the yeasts. By using a single type of yeast cells (MATa), we can artificially add α factor to trigger the yeasts to grow towards imaginary partners (poor yeast!), which will activate the mating pathway. This procedure gives us greater control over the experiment to discover the function of similar pathways in humans.
Cells, including those of yeasts and humans, are like life factories. DNA is similar to a manager that controls the production: various types of proteins, which are made by different combinations of amino acids following instructions (mRNA). However, in both human and yeast’s cells, DNA usually stays in the nucleus and does not directly respond to outside changes. Therefore, little messengers on the cell membrane (receptors) need to find a way to inform the cell about certain changes, such as noticing the opposite sex.
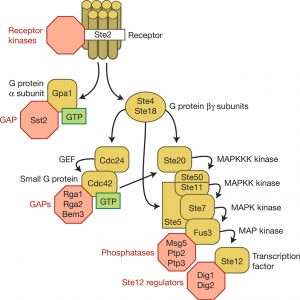
Similarly, receptors often cannot leave the membrane as well, so they usually pass the information through a chain of signaling events using proteins, such as kinases (another type of messengers). For example, in the yeast mating pathway, the pheromone binds with receptors on the surface of the cell, a series of responses are triggered and will result in the changing of expression of the yeast gene.
In human cells, once the pathway for receiving information is messed up, cancer might grow.
Thus, finding out how signals are passed or received in yeast cells may tell us more about the origin of cancers and even provide a solution. There are multiple kinases linked to each other that change protein production in response to outside signals as shown in Figure 1. According to Wang and Dohlman, this signaling pathway in yeast is similar to those in human cells, which makes yeast a great tool for understanding our own cells.
The cell must change constantly to fit the environment, which means the signaling pathway can also be regulated. For example, Dr. Dohlman explained that as you drink more and more coffee, you may find its effect diminishing, because the receptors are saturated with the stimulus (they are too busy to manage more messages), which is the result of desensitization.
Problem with pathway regulation is one of the reasons for messed up signaling that may cause cancers. Once we find out the function for each component of certain pathways in yeast, we may apply the results on human cells to find the targets in the problematic pathways.
Although it was mentioned above that DNA, the manager, does not usually respond inside the cell, histone methylation is an exception of pathway regulation. DNA is like a long, thin wire, so it needs to be organized and packed. Histone is a cube-like protein that allows DNA to twist around it in order to form chromosomes. The methylation of histone will add methyl groups, like little arms, to the histone, which will make the DNA wraps tighter or looser. Thus, the production of “the factory” will increase or decrease depending on where the methyl groups are added.
In human cells, SETD2 gene provides instructions for methyltransferases, which can perform such gene regulation. The mutation in SETD2 may lead to clear cell renal cell carcinoma (cRCC), a type of kidney cancer, according to Duns, or high-grade gliomas (HGGs), a type of brain tumor, based on Fontebasso’s result.
In yeast, SET2 gene codes for Set2 protein, the only methyltransferase that has such function. SET2 is like a close cousin of SETD2. Thus, studying how SET2 may regulate the mating pathway can shed light on the function of SETD2 in human cells that may provide a potential solution for drug targets.
Yeast has been studied as a standard model for years, which means we know all of its gene and numerous ways to manipulate different parts of its gene. It is a common practice for geneticists to remove or add certain genes into the yeast, just like LEGO pieces, to study the functions of genes.
For the purpose of studying the function of SET2, we can simply remove the SET2 in yeasts, grow them, and compare them with normal yeasts. In contrast with clinical trials or experiment on human cells, which usually take months or even years, it only takes less than a week for the yeast to show the influences of the missing gene.
Besides, with one single experiment, there are about 10,000,000,000 (ten billion) yeast cells involved, which are more than the combination of human’s population of the entire planet. This great number of samples provide an advantage for a more accurate result. We can now easily monitor the difference in mating pathway with the removal of SET2 to figure out how it influences the signaling pathway.
With the help of yeasts, scientists can solve human medical mysteries more efficiently. So please do not treat yeasts like magical little creatures that can only enrich your dinner; they are pioneers for human health research. Yes, yeast matters, and it matters more than you think.
By: H. Wang
References:
Dohlman, H. G. (2002). Desensitization: Diminishing returns. Nature, 418(6898), 591-591.
Duns, G., van den Berg, E., van Duivenbode, I., Osinga, J., Hollema, H., Hofstra, R. M., & Kok, K. (2010). Histone methyltransferase gene SETD2 is a novel tumor suppressor gene in clear cell renal cell carcinoma. Cancer research, 70(11), 4287-4291.
Fontebasso, A. M., Schwartzentruber, J., Khuong-Quang, D. A., Liu, X. Y., Sturm, D., Korshunov, A., … & Fleming, A. (2013). Mutations in SETD2 and genes affecting histone H3K36 methylation target hemispheric high-grade gliomas. Acta neuropathologica, 125(5), 659-669.
Wang, Y., & Dohlman, H. G. (2004). Pheromone signaling mechanisms in yeast: a prototypical sex machine. Science, 306(5701), 1508-1509.
Image Credits:
Wang, H., “Yeast Matters”
Wang, Y., & Dohlman, H. G., “Pheromone signaling mechanisms in yeast: a prototypical sex machine”